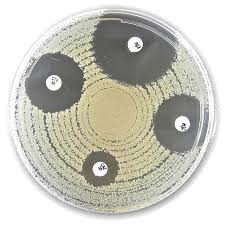
Liofilchem® Tetracycline TE, Antimicrobial Susceptibility Discs, 30 µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Tetracycline (TE) Antimicrobial Susceptibility Discs (30 µg) are high-quality, sterile paper discs used for in vitro antimicrobial susceptibility testing (AST) of bacteria using the Kirby-Bauer disk diffusion method. Each disc is impregnated with 30 micrograms of tetracycline, a broad-spectrum antibiotic belonging to the tetracycline class, which inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit.
These discs are manufactured in compliance with ISO 9001 and ISO 13485 standards, ensuring precision, reproducibility, and consistency in zone of inhibition results. They are widely used in clinical, veterinary, food safety, and pharmaceutical microbiology laboratories.
Key Features
- Standardized potency: Each disc delivers 30 µg of tetracycline to enable accurate susceptibility profiling.
- Broad-spectrum activity: Effective against a wide range of Gram-positive and Gram-negative bacteria, including Staphylococcus aureus, Escherichia coli, Enterococcus spp., Salmonella spp., and Vibrio cholerae.
- AST guideline compliant: Interpretable using CLSI or EUCAST zone diameter breakpoints.
- Sterile and stable: Supplied in sealed cartridges (50 discs) or canisters (250 discs) with desiccant for long-term shelf stability.
- Color-coded labeling: For clear identification and efficient use in high-throughput testing workflows.
Applications and Use Cases
1. Clinical Microbiology
- Detection of tetracycline susceptibility in urinary tract, respiratory, and enteric pathogens.
- Resistance profiling of Staphylococcus spp., Streptococcus spp., and Enterobacteriaceae.
2. Veterinary Diagnostics
- AST of livestock- and poultry-associated pathogens such as Pasteurella multocida, Actinobacillus spp., and Mycoplasma spp.
3. Food and Water Testing
- Surveillance of tetracycline resistance in zoonotic bacteria such as Salmonella and Campylobacter from food and environmental samples.
4. Pharmaceutical and AMR Research
- Comparative studies with other tetracyclines (e.g., doxycycline, oxytetracycline).
- Monitoring resistance mechanisms including tet genes in surveillance programs.
Use Instructions
- Medium: Mueller-Hinton Agar (MHA) is the recommended base medium.
- Inoculum Preparation: Standardize the bacterial suspension to 0.5 McFarland turbidity.
- Inoculation: Evenly swab the entire agar surface with the standardized inoculum.
- Disc Application: Place one 30 µg tetracycline disc using sterile forceps or a mechanical disc dispenser.
-
Incubation:
- Temperature: 35 ± 2 °C
- Time: 16–18 hours, under aerobic conditions.
-
Interpretation:
- Measure the diameter of the inhibition zone in millimeters.
- Compare with CLSI or EUCAST breakpoints for tetracycline.
Technical Specifications
| Specification | Detail |
|---|---|
| Antibiotic | Tetracycline (TE) |
| Disc Potency | 30 µg |
| Disc Diameter | 6 mm |
| Format | Sterile filter paper disc |
| Packaging | 50 discs (cartridge) / 250 discs (canister) |
| Storage Conditions | 2–8 °C (refrigerated, dry, sealed) |
| Shelf Life | 24–36 months from date of manufacture |
Quality Control
Tested with standard quality control organisms under CLSI/EUCAST protocols:
| Control Organism | Expected Zone Diameter |
|---|---|
| Escherichia coli ATCC® 25922 | CLSI/EUCAST compliant |
| Staphylococcus aureus ATCC® 25923 | CLSI/EUCAST compliant |
| Enterococcus faecalis ATCC® 29212 | CLSI/EUCAST compliant |
Liofilchem® Tetracycline TE Discs (30 µg) offer a reliable, cost-effective solution for evaluating the antimicrobial susceptibility of a wide range of clinically and environmentally important bacteria. With ISO-certified quality, standardized potency, and broad-spectrum activity, these discs are ideal for AST in clinical, veterinary, public health, and research settings.




 0
0