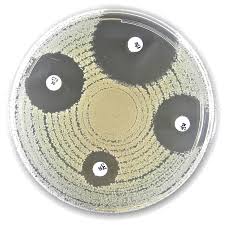
Liofilchem® Nalidixic acid NA, Antimicrobial Susceptibility Discs, 30 µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Nalidixic Acid (NA) Antimicrobial Susceptibility Discs (30 µg) are high-quality, ready-to-use paper discs designed for the in vitro assessment of bacterial susceptibility to nalidixic acid using the Kirby-Bauer disk diffusion method. Each disc is impregnated with 30 micrograms of nalidixic acid, a synthetic quinolone antibiotic primarily used for testing susceptibility in Enterobacteriaceae, Escherichia coli, Shigella, and other Gram-negative organisms.
These discs are manufactured under stringent ISO 9001 and ISO 13485 quality management systems, ensuring reproducibility, sterility, and compliance with international standards for clinical microbiology and diagnostic laboratories.
Key Features
- Accurate dosage: Each disc delivers a consistent 30 µg dose of nalidixic acid for reliable inhibition zone measurement.
- Validated for Gram-negative bacteria: Particularly effective against E. coli, Proteus spp., and Salmonella spp.
- Standardized for clinical use: Designed for disk diffusion method (Kirby-Bauer), compliant with CLSI and EUCAST guidance.
- Sterile and stable: Packed with desiccant in sealed cartridges or canisters to maintain potency during storage and use.
- Color-coded labeling: Simplifies disc identification and workflow management in high-throughput laboratories.
Applications and Use Cases
1. Clinical Microbiology
- Routine susceptibility testing of urinary tract pathogens like E. coli and Proteus mirabilis.
- Screening for nalidixic acid resistance in Salmonella spp. to infer ciprofloxacin susceptibility.
2. Public Health and Food Safety
- Used in foodborne pathogen surveillance for Shigella and Salmonella resistance profiling.
- Epidemiological studies of quinolone resistance in Gram-negative bacteria.
3. Veterinary Microbiology
- Susceptibility testing of animal-derived enteric isolates from livestock and companion animals.
4. Antibiotic Development & Research
- Comparative zone of inhibition testing alongside fluoroquinolones like ciprofloxacin and norfloxacin.
Use Instructions
- Prepare the medium: Mueller-Hinton Agar (MHA) is recommended.
- Inoculum standardization: Adjust bacterial suspension to 0.5 McFarland turbidity.
- Inoculation: Use a sterile swab to evenly spread the inoculum on the agar surface.
- Disc placement: Apply the 30 µg nalidixic acid disc to the agar using sterile forceps or a dispenser.
- Incubation: 35 ± 2 °C for 16–18 hours in ambient air.
- Interpretation: Measure the diameter of the inhibition zone and compare with CLSI/EUCAST interpretive criteria.
Storage and Shelf Life
- Storage Temperature: 2–8 °C (Refrigerated, with desiccant)
- Shelf Life: Typically 24–36 months from manufacture when stored properly
-
Packaging Options:
- Cartridges: 50 discs
- Canisters: 250 discs
Quality Assurance
- Manufactured under ISO 9001 and ISO 13485 certification
- Batch-to-batch consistency ensured by rigorous QC using standard reference strains
-
Typical test organisms:
- E. coli ATCC 25922 (susceptible control)
- Proteus mirabilis ATCC 12453 (for QC verification)
Liofilchem® Nalidixic Acid NA Discs (30 µg) provide a precise, standardized method for evaluating the effectiveness of nalidixic acid against Gram-negative bacteria. Ideal for routine diagnostics, resistance monitoring, and surveillance applications, they are a trusted choice for microbiology labs adhering to CLSI/EUCAST standards. Their performance, stability, and accuracy make them a critical component in antimicrobial susceptibility workflows.




 0
0