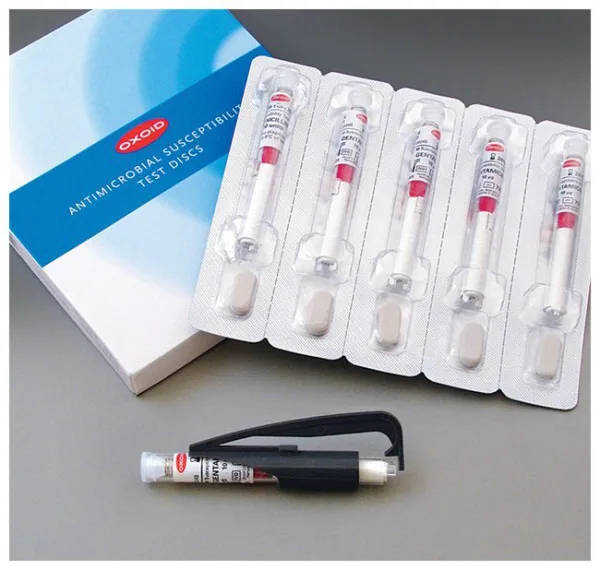
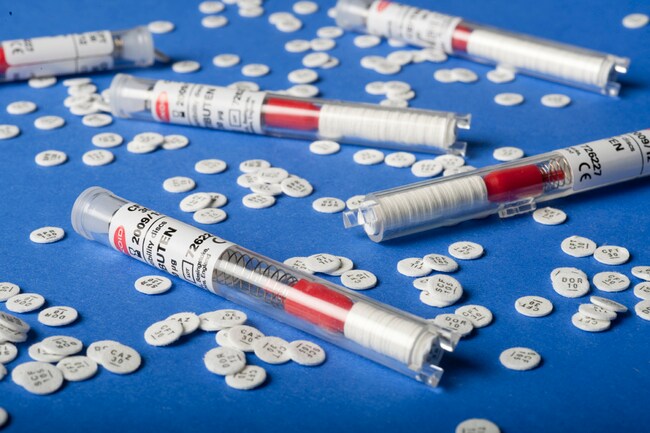
Thermo Scientific™ Oxoid™ Cefixime CFM Antimicrobial Susceptibility discs
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | -20°C |
| Product Type | Antibiotic Disc |
| Product Brand | Thermo Fisher Scientific™, Oxoid |
| Product Grade | Microbiology grade |
Thermo Scientific™ Oxoid™ Cefixime Antimicrobial Susceptibility Discs provide a reliable and standardized method for determining the in vitro susceptibility of microorganisms to cefixime using manual disc diffusion (Kirby-Bauer) techniques. These discs are pre-loaded with 5 µg of cefixime—a third-generation oral cephalosporin with broad-spectrum activity against Gram-negative bacteria including Neisseria gonorrhoeae, Haemophilus influenzae, and select Enterobacteriaceae.
Designed for use in routine antimicrobial resistance screening and clinical diagnostics, each disc is housed in a spring-loaded, shatterproof cartridge system with easy-to-read labeling and desiccant protection to maintain a moisture level below 2%—ensuring long-term stability and optimal performance.
Key Features & Benefits
| Feature | Description |
|---|---|
| Consistent Potency | Contains 5 µg of cefixime for standardized disc diffusion testing |
| Reliable Detection | Categorizes resistant, intermediate, and susceptible isolates |
| Enhanced Interpretation | Allows visual detection of resistant mutants, inoculum issues, and antagonism |
| Validated Design | Individually desiccated cartridges ensure moisture control and long-term storage |
| Ease of Use | Spring-loaded cartridges support hygienic, single-disc dispensing |
| International Compliance | Complies with CLSI, EUCAST, and BSAC testing guidelines |
Intended Use
Thermo Scientific™ Oxoid™ Cefixime Discs are intended for in vitro diagnostic use in semi-quantitative antimicrobial susceptibility testing by disc diffusion methods. These discs are used with a pure agar-grown bacterial isolate to guide therapeutic decisions, particularly in the treatment of infections caused by cefixime-susceptible pathogens. They are suitable for use in hospital microbiology labs, clinical research settings, and antimicrobial resistance surveillance programs.
Technical Specifications
| Attribute | Detail |
|---|---|
| Antibiotic | Cefixime |
| Antibiotic Code | CFM |
| Disc Potency | 5 µg |
| Format | 50 Discs/Cartridge, 5 Cartridges/Pack |
| Packaging Type | Shatterproof, spring-loaded cartridges |
| Moisture Control | <2% (with desiccant seal) |
| Test Method | Disc diffusion (Kirby-Bauer) |
| Regulatory Status | For In Vitro Diagnostic Use |
| Certifications/Compliance | CLSI, BSAC, EUCAST |
| Application | Clinical microbiology, AST, resistance profiling |
| Unit Size | Each |
Applications
-
Clinical Diagnostics
Determine susceptibility of pathogens in urinary tract infections, respiratory infections, and sexually transmitted diseases. -
Public Health and AMR Surveillance
Support resistance monitoring and epidemiological studies. -
Research Laboratories
Evaluate novel combination therapies and track emerging resistance patterns.
Thermo Scientific™ Oxoid™ Cefixime 5 µg Susceptibility Discs deliver high-quality, reproducible results in antimicrobial susceptibility testing workflows. Trusted in laboratories worldwide, they meet international standards and offer convenient, stable packaging for reliable daily use. An essential diagnostic tool in the detection and management of cefixime-sensitive bacterial infections.
- Drug Concentration: 5 µg 10 µg




 0
0