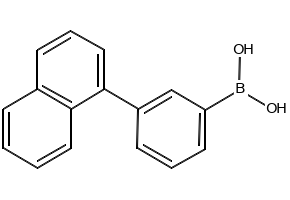
Thermo Scientific™ (3-(Naphthalen-1-yl)phenyl)boronic acid 98%
Catalog No :
CAS Number :
Brand :
In Stock
Formula: C₁₆H₁₃BO₂
Storage Temperature: Ambient
CAS Number: 881913-20-8
Specifications:
| Application | Organic Synthesis | ||
| Storage Temperature | Ambient | ||
| Product Type | Laboratory Chemical | Forms | Powder |
| Product Brand | Thermo Fisher Scientific™, thermo Scientific | ||
| Product Grade | Analytical grade | Formula | C₁₆H₁₃BO₂ |
(3-(Naphthalen-1-yl)phenyl)boronic acid is a highly pure arylboronic acid derivative used in Suzuki-Miyaura cross-coupling reactions and other palladium-catalyzed coupling strategies for building complex aromatic and heteroaromatic compounds. Featuring both naphthalene and phenyl moieties, this compound is suitable for use in pharmaceutical intermediate synthesis, organic electronics, materials science, and medicinal chemistry research.
This reagent is supplied at ≥98% purity, making it suitable for demanding synthetic applications where chemical precision is critical. It is compatible with most common organometallic reagents and reaction conditions.
Key Features
- High purity: ≥98%
- Supplied as a white crystalline powder
- Ideal for Suzuki coupling and C–C bond formation
- Useful in medicinal chemistry, OLED/organic materials synthesis, and combinatorial libraries
- Stable under standard laboratory storage conditions
- Available in 250 mg, 1 g, and 5 g quantities
Applications
- Suzuki-Miyaura cross-coupling for C–C bond formation
- Synthesis of biaryls, pharmaceutical intermediates, and aromatic frameworks
- Ligand and catalyst development
- Organic material synthesis (e.g., OLEDs, sensors)
- Research in medicinal and synthetic organic chemistry
Chemical & Physical Properties
| Property | Specification |
|---|---|
| Chemical Name | (3-(Naphthalen-1-yl)phenyl)boronic acid |
| Synonyms | 3-naphthalen-1-yl phenylboronic acid, 3-(1-naphthyl)benzeneboronic acid, etc. |
| CAS Number | 881913-20-8 |
| Molecular Formula | C₁₆H₁₃BO₂ |
| Molecular Weight | 248.09 g/mol |
| Percent Purity | ≥98% |
| Physical Form | White powder |
| Color | White |
| MDL Number | MFCD11045054 |
| PubChem CID | 58881077 |
| IUPAC Name | (3-naphthalen-1-ylphenyl)boronic acid |
| SMILES | B(C1=CC(=CC=C1)C2=CC=CC3=CC=CC=C32)(O)O |
| InChI Key | HFXYUCJLQZCNPD-UHFFFAOYSA-N |
Available Packaging Sizes
| Catalog Number | Quantity | Packaging |
|---|---|---|
| H64019.MD | 250 mg | Glass vial |
| H64019.03 | 1 g | Glass vial |
| H64019.06 | 5 g | Glass vial |
Thermo Scientific™ (3-(Naphthalen-1-yl)phenyl)boronic acid, 98% is a versatile, high-purity reagent essential for modern organic synthesis and materials science. Its high purity and reliable performance make it an excellent choice for researchers involved in cross-coupling reactions, small molecule synthesis, and functional material development.
- Pack Size: 250mg 1g 5g




 0
0