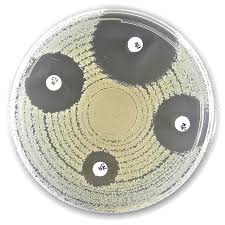
Liofilchem® Ampicillin AMP, Antimicrobial Susceptibility Discs, 10 µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Antimicrobial Susceptibility and Resistance Testing (AST) |
| Storage Temperature | -20°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Ampicillin (AMP) Antimicrobial Susceptibility Discs are precision diagnostic tools designed for the determination of bacterial susceptibility to ampicillin. Available in concentrations of 2 µg and 10 µg, these discs are used in standardized disc diffusion assays, conforming to CLSI (Clinical and Laboratory Standards Institute) and EUCAST (European Committee on Antimicrobial Susceptibility Testing) guidelines. These discs are essential in clinical microbiology laboratories for determining bacterial resistance patterns and aiding in the selection of effective antibiotic treatments.
Key Features:
- High Precision: Each disc is impregnated with a defined and uniform concentration of ampicillin (2 µg or 10 µg) to ensure reproducible results.
- Quality Manufacturing: Produced under strict quality control to meet international standards (CLSI/EUCAST) for antimicrobial susceptibility testing.
- Wide Application: Effective against a range of Gram-positive and Gram-negative bacterial pathogens.
- Easy Handling: Packaged in convenient cartridges compatible with Liofilchem® disc dispensers for accurate placement on agar surfaces.
Intended Use:
- Bacterial Susceptibility Testing: The discs are used to evaluate the susceptibility of bacterial isolates to ampicillin using the Kirby-Bauer disc diffusion method on Mueller-Hinton Agar.
- Clinical Decision Support: Results guide clinicians in selecting effective antibiotic therapy for infections caused by susceptible bacteria, including Escherichia coli, Salmonella spp., Enterococcus spp., and Proteus mirabilis.
Test Procedure:
- Preparation:
- Inoculate the Mueller-Hinton Agar plate with a standardized bacterial suspension (0.5 McFarland standard).
- Spread the inoculum evenly across the plate to ensure a uniform lawn of bacterial growth.
- Application:
- Place the Ampicillin Antimicrobial Susceptibility Discs (2 µg or 10 µg) onto the agar surface using a sterile forceps or a Liofilchem® disc dispenser.
- Ensure proper spacing between discs to prevent overlapping zones of inhibition.
- Incubation:
- Incubate the plate aerobically at 35 ± 2°C for 16–18 hours.
- Interpretation:
- Measure the diameter of the inhibition zones using a calibrated ruler or zone reader.
- Compare the zone diameters to interpretive standards provided by CLSI or EUCAST.
Quality Control:
To ensure accuracy and reliability, quality control must be performed using standard bacterial strains, such as:
- Escherichia coli ATCC® 25922: Ampicillin-susceptible control strain.
- Staphylococcus aureus ATCC® 25923: Ampicillin-resistant control strain.
Key Applications:
- Clinical Microbiology: Diagnosis of bacterial infections and determination of resistance.
- Public Health Surveillance: Monitoring antimicrobial resistance trends.
- Pharmaceutical Research: Evaluation of ampicillin efficacy in drug development.
Storage Conditions:
- Store the discs at 2–8°C in their original sealed container.
- Avoid exposure to moisture, light, and temperature fluctuations.
- Use before the expiry date indicated on the packaging to ensure optimal performance.
Compliance and Standards:
- CLSI and EUCAST Guidelines: Validated to meet international standards for antimicrobial susceptibility testing.
- ISO Certification: Manufactured in ISO-certified facilities to ensure consistency and quality.
Packaging Information:
- Disc Concentrations: Available in 2 µg and 10 µg strengths.
- Cartridge Size: Supplied in cartridges containing 50 discs per pack, compatible with automated and manual disc dispensers.
Precautions:
- For in vitro diagnostic use only.
- Perform the test under aseptic conditions to prevent contamination.
- Results should be interpreted by trained professionals in conjunction with clinical data and other laboratory findings.
Limitations:
- Not suitable for anaerobic organisms or slow-growing bacteria that require specialized media.
- Ensure adherence to standardized protocols for accurate and reproducible results.
- Resistance mechanisms such as beta-lactamase production may impact susceptibility outcomes.
References:
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing (latest edition).
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters (current version).
The Liofilchem® Ampicillin (AMP) Antimicrobial Susceptibility Discs are essential tools for modern microbiology laboratories, ensuring accurate detection of bacterial resistance and aiding in effective treatment strategies.
- Drug Concentration: 2 µg 10 µg




 0
0