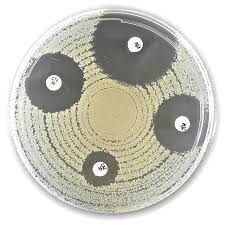
Liofilchem Piperacillin PRL 30 µg, 5x50 Disc
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Piperacillin Antimicrobial Susceptibility Discs (30 µg) are designed for use in antibiotic susceptibility testing (AST) to determine the sensitivity or resistance of bacterial isolates to Piperacillin, a broad-spectrum penicillin antibiotic. These discs are an essential tool in clinical microbiology laboratories, aiding in the selection of appropriate antimicrobial therapy for bacterial infections.
Key Features:
- Standardized Dosage: Each disc contains 30 µg of Piperacillin, ensuring consistent and reliable results.
- High-Quality Manufacturing: Made under stringent quality control to comply with global standards such as EUCAST and CLSI guidelines.
- Sterile and Ready-to-Use: Packaged with a desiccant to maintain integrity and potency.
- Precision Testing: Facilitates differentiation between susceptible, intermediate, and resistant bacterial strains.
Composition:
- Active Ingredient: 30 µg of Piperacillin impregnated in each disc.
- Disc Material: High-quality, absorbent paper.
Method Principle:
The disc diffusion method (Kirby-Bauer technique) is employed:
- Discs are placed on the surface of an agar plate inoculated with the test organism.
- The plate is incubated, allowing the antibiotic to diffuse into the agar.
- Zones of inhibition (areas with no bacterial growth) are measured to determine susceptibility.
Applications and Use Cases:
1. Clinical Diagnosis:
- Target Pathogens: Effective against a wide range of Gram-negative bacteria (e.g., Escherichia coli, Pseudomonas aeruginosa, Klebsiella spp.) and some Gram-positive bacteria.
- Use Case:
- Determine the susceptibility of bacteria isolated from clinical specimens such as:
- Blood cultures
- Urine samples (for urinary tract infections)
- Wound swabs
- Respiratory samples (e.g., sputum)
- Guide therapeutic decisions in infections caused by Pseudomonas aeruginosa, a common nosocomial pathogen.
- Determine the susceptibility of bacteria isolated from clinical specimens such as:
2. Monitoring Antimicrobial Resistance:
- Used in antimicrobial stewardship programs to track resistance patterns over time.
- Aids in evaluating hospital-acquired infections and determining shifts in microbial resistance trends.
3. Veterinary and Environmental Testing:
- Applied in testing bacterial isolates from animals, food products, or environmental samples to monitor contamination and resistance development.
Interpretation of Results:
The zone of inhibition (in millimeters) is measured and interpreted as:
- Sensitive (S): Indicates the organism is inhibited by Piperacillin at standard dosages.
- Intermediate (I): Indicates possible reduced susceptibility; higher doses may be required.
- Resistant (R): Indicates the organism is not inhibited and alternative antibiotics are required.
Interpretation criteria align with EUCAST or CLSI standards.
Quality Control:
Control strains are tested periodically to ensure consistent performance:
- Escherichia coli ATCC® 25922: Expected inhibition zone within the specified range.
- Pseudomonas aeruginosa ATCC® 27853: Used to validate effectiveness against challenging Gram-negative bacteria.
- Staphylococcus aureus ATCC® 25923: Confirms absence of cross-resistance.
Storage Conditions:
- Store at 2-8°C in a dry environment away from direct sunlight.
- Avoid freezing or exposure to high humidity to maintain disc potency.
- Use before the expiry date printed on the packaging.
Packaging:
- Discs are available in cartridges containing 50 or 100 discs, individually sealed with a desiccant to maintain activity.
Advantages:
- Broad Spectrum: Covers diverse bacterial pathogens, reducing empirical treatment errors.
- Standardized Results: Facilitates uniformity and reproducibility across laboratories.
- Cost-Effective: Enables simultaneous testing of multiple isolates.
- Global Compliance: Meets international standards for antimicrobial susceptibility testing.
Precautions:
- Handle with aseptic techniques to prevent contamination.
- Use only with pure bacterial cultures.
- Ensure proper incubation conditions (e.g., Mueller-Hinton agar at 35°C) for accurate results.
- Discs are for in vitro diagnostic use only.
Summary of Use Cases:
- Healthcare Settings: Diagnosis and treatment of bacterial infections.
- Public Health Surveillance: Monitoring antibiotic resistance trends.
- Veterinary Labs: Ensuring proper treatment for animal infections.
- Environmental Testing: Evaluating contamination in water or food industries.
Product Specifications:
- Product Name: Liofilchem® Piperacillin Antimicrobial Susceptibility Discs
- Dosage: 30 µg per disc
- Model Number: Specific to the product batch
- Shelf Life: 2-3 years under recommended storage conditions
- Compliance: EUCAST and CLSI standards
By providing consistent, reliable results, Liofilchem Piperacillin Antimicrobial Susceptibility Discs play a pivotal role in optimizing antimicrobial use and combating bacterial resistance.




 0
0