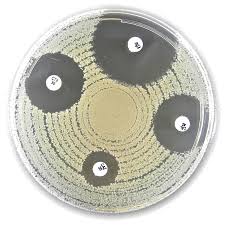
Liofilchem® Ampicillin-sulbactam AMS Antimicrobial Susceptibility Discs, 20 µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
Liofilchem® Ampicillin-Sulbactam (AMS) Antimicrobial Susceptibility Discs, 20 µg, are diagnostic tools used in antimicrobial susceptibility testing (AST) to determine the sensitivity of bacterial pathogens to the combination of ampicillin and sulbactam. These discs are designed for use in clinical microbiology laboratories and are essential for identifying effective antibiotic regimens, particularly for infections caused by beta-lactamase-producing bacteria.
Key Features
- Dual-Drug Combination: Each disc contains ampicillin and sulbactam in a fixed 2:1 ratio, ensuring effective inhibition of beta-lactamase-producing bacteria.
- Dosage: Discs contain 20 µg of the combination, providing a reliable concentration for susceptibility testing.
- Regulatory Compliance: Manufactured in compliance with CLSI and EUCAST guidelines to ensure accurate and reproducible results.
- High-Quality Standards: Consistent performance in testing with stringent quality control.
Applications
- Clinical Microbiology:
- Evaluating the susceptibility of Gram-negative bacteria such as Escherichia coli, Klebsiella spp., Acinetobacter spp., and Pseudomonas aeruginosa.
- Testing beta-lactamase-producing bacteria to assess the efficacy of ampicillin-sulbactam therapy.
- Antimicrobial Resistance Monitoring:
- Supporting the surveillance of resistance trends in hospital and community-acquired infections.
- Research and Development:
- Utilized in studies of antimicrobial efficacy and resistance mechanisms in microbiology research laboratories.
Test Procedure
- Preparation:
- Prepare Mueller-Hinton Agar (MHA) following standard protocols.
- Adjust the bacterial suspension to a 0.5 McFarland standard.
- Application:
- Using sterile forceps or a disc dispenser, place the 20 µg AMS disc on the agar surface inoculated with the bacterial suspension.
- Ensure the disc is in full contact with the agar.
- Incubation:
- Incubate the plates at 35 ± 2°C in an aerobic environment for 16–20 hours.
- Result Interpretation:
- Measure the diameter of the inhibition zone in millimeters (mm) around the disc.
- Compare the measured diameter with breakpoint tables provided by CLSI or EUCAST to determine susceptibility (S), intermediate (I), or resistance (R).
Quality Control
The performance of the AMS discs is validated using the following quality control strains:
- Escherichia coli ATCC® 25922 – Expected zone diameter within established ranges.
- Klebsiella pneumoniae ATCC® 700603 – Beta-lactamase positive control.
- Pseudomonas aeruginosa ATCC® 27853 – Beta-lactamase negative control.
Storage and Stability
- Store at 2–8°C in a dry environment, protected from light and moisture.
- Use before the expiration date printed on the label.
- Avoid exposure to heat or humidity to maintain disc potency.
Precautions
- For in vitro diagnostic use (IVD) only.
- Handle with sterile forceps to prevent contamination.
- Dispose of used discs and plates in accordance with biosafety and institutional guidelines.
Regulatory Compliance
- Manufactured to meet the standards outlined by ISO 11133, CLSI, and EUCAST for antimicrobial susceptibility testing.
References
- Clinical and Laboratory Standards Institute (CLSI) guidelines.
- EUCAST breakpoint interpretations.
- Manufacturer’s technical documentation for Liofilchem® AMS discs.
Key Benefits
- Reliable detection of beta-lactam/beta-lactamase inhibitor activity.
- Effective tool for monitoring antibiotic resistance trends.
- Supports clinical decision-making in antimicrobial therapy for challenging infections.




 0
0