Liofilchem® Chloramphenicol C, Antimicrobial Susceptibility Discs, 30 µg
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Clinical microbiology |
| Storage Temperature | 2-8°C |
| Product Type | Antibiotic Disc |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
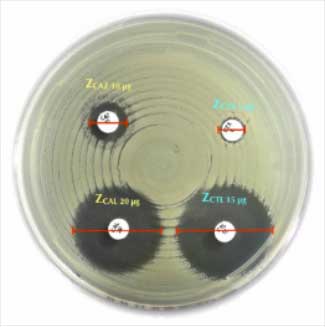
Liofilchem® Chloramphenicol Antimicrobial Susceptibility Discs, available in 10 µg and 30 µg concentrations, are essential tools for determining bacterial susceptibility to chloramphenicol via the disk diffusion method. This method aids in guiding effective antimicrobial therapy and monitoring resistance patterns.
Liofilchem® Chloramphenicol Antimicrobial Susceptibility Discs
- Concentrations: 10 µg and 30 µg
- Purpose: Determination of bacterial susceptibility to chloramphenicol
- Methodology: Disk diffusion (Kirby-Bauer method)
Test Procedure
1. Inoculum Preparation
- Step:
- Select well-isolated colonies from overnight culture.
- Suspend colonies in sterile saline or broth to a turbidity equivalent to 0.5 McFarland standard.
- Quality Note:
- Use a densitometer to ensure accurate turbidity measurement.
2. Inoculation of Agar Plate
- Step:
- Dip a sterile swab into the prepared inoculum suspension.
- Remove excess by pressing against the tube wall.
- Evenly streak across a Mueller-Hinton agar plate for a uniform lawn of growth.
- Key Tip:
- Rotate the plate 60° between streaks for consistent distribution.
3. Application of Chloramphenicol Discs
- Step:
- Allow the plate to dry for 3–5 minutes at room temperature.
- Using sterile forceps or a disc dispenser, apply chloramphenicol discs evenly on the agar.
- Spacing Note:
- Maintain sufficient space between discs to prevent overlapping inhibition zones.
4. Incubation
- Step:
- Invert and incubate at 35 ± 2°C for 16–18 hours under ambient conditions.
5. Reading and Interpretation
- Step:
- Measure inhibition zones to the nearest millimeter.
- Compare results to CLSI/EUCAST breakpoint standards.
Quality Control
Recommended QC Strains and Zone Diameter Ranges
| Organism | ATCC® Number | Expected Zone Diameter (mm) |
|---|---|---|
| Escherichia coli | ATCC® 25922 | 21–27 |
| Staphylococcus aureus | ATCC® 25923 | 18–24 |
| Pseudomonas aeruginosa | ATCC® 27853 | 19–25 |
Quality Control Guidelines
- Perform QC weekly or with each new batch of discs.
- Investigate deviations in zone diameters:
- Verify inoculum density.
- Check agar depth (4 mm is standard).
- Confirm disc potency and storage conditions (2–8°C).
Comparison with Other Brands
| Feature | Liofilchem® | Oxoid™ | BD BBL™ |
|---|---|---|---|
| Disc Potency Options | 10 µg, 30 µg | 30 µg | 30 µg |
| Compliance | CLSI/EUCAST | CLSI/EUCAST | CLSI/EUCAST |
| Cartridge Design | Standard | Spring-loaded | Standard |
| Packaging | 50 discs/cartridge, 5 packs | 50 discs/cartridge, 5 packs | 50 discs/cartridge, 10 packs |
Interpretation Guidelines
- CLSI Breakpoints for Chloramphenicol:
- Susceptible (S): ≥ 18 mm
- Intermediate (I): 13–17 mm
- Resistant (R): ≤ 12 mm
References
- Liofilchem® Antibiotic Disc Instructions for Use:
Liofilchem Instructions PDF - CLSI Performance Standards:
CLSI Guidelines - EUCAST Breakpoints:
EUCAST Guidelines
- Drug Concentration: 30 µg 10 µg




 0
0