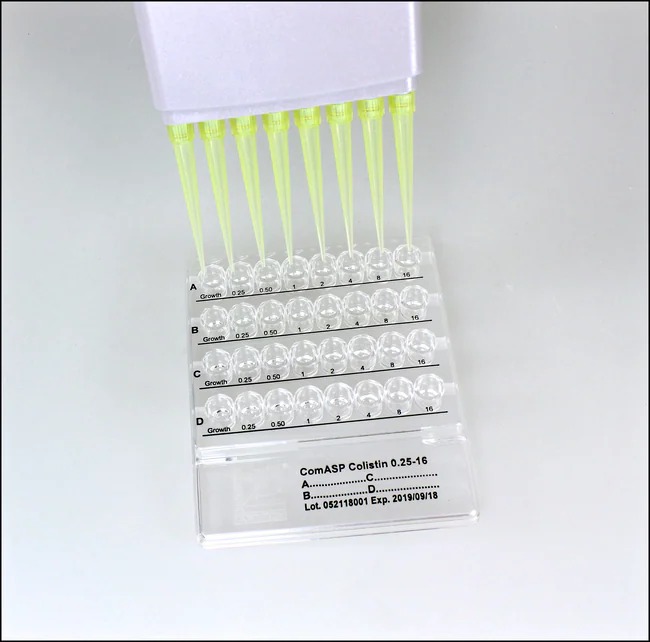
Liofilchem® ComASP™ Benzylpenicillin 0.002-32µg/ml, 12 tests
Catalog No :
CAS Number :
Brand :
In Stock
Specifications:
| Application | Clinical microbiology |
| Storage Temperature | -20°C |
| Product Type | Microdilution Panel |
| Product Brand | Liofilchem |
| Product Grade | Microbiology grade |
The Liofilchem® ComASP™ Benzylpenicillin MIC Broth Microdilution Panel is designed for the accurate determination of minimum inhibitory concentrations (MICs) for Benzylpenicillin across a range of 0.002-32 µg/mL. It provides reliable, standardized results to assist in the detection of antimicrobial susceptibility or resistance in clinical and research laboratories. This product is suitable for testing against key pathogens, including Streptococcus pneumoniae, Neisseria gonorrhoeae, and other gram-positive bacteria.
Features and Benefits
- Comprehensive MIC Range: Covers a wide MIC range from 0.002 to 32 µg/mL, suitable for detecting varying levels of bacterial susceptibility to Benzylpenicillin.
- Ready-to-Use Panels: Pre-prepared panels eliminate the need for manual preparation, reducing errors and saving time.
- Standardized Format: Conforms to international guidelines (CLSI and EUCAST), ensuring globally accepted results.
- Validated for Clinical Pathogens: Optimized for use with bacterial strains commonly associated with respiratory infections, meningitis, and sexually transmitted infections.
- Cost-Effective: Provides 12 tests per kit, making it an efficient choice for small-to-medium scale laboratories.
- Storage Stability: Panels are stable for long-term storage at -20°C and short-term storage at 2–8°C.
Specifications
| Attribute | Details |
|---|---|
| Antibiotic Tested | Benzylpenicillin |
| MIC Range | 0.002–32 µg/mL |
| Test Format | Broth microdilution |
| Number of Tests | 12 per kit |
| Storage Conditions | -20°C for long-term, 2–8°C for short-term |
| Intended Use | Research and clinical use for susceptibility testing |
| Regulatory Compliance | ISO 20776-1, CLSI M07, and EUCAST standards |
Test Procedures
- Preparation:
- Allow the panel to equilibrate to room temperature.
- Prepare bacterial suspensions in accordance with CLSI or EUCAST guidelines.
- Inoculation:
- Add the standardized inoculum (as per guidelines) to the wells containing Benzylpenicillin concentrations.
- Ensure proper sealing of the panel to avoid evaporation.
- Incubation:
- Incubate at 35°C ± 2°C for 16–20 hours for most pathogens.
- Interpretation:
- Observe the wells for visible bacterial growth.
- The MIC is defined as the lowest concentration of Benzylpenicillin that prevents visible growth.
Quality Control
| Organism | MIC Range (µg/mL) | Reference Standard |
|---|---|---|
| Streptococcus pneumoniae ATCC® 49619 | 0.016–0.064 | EUCAST/CLSI |
| Neisseria gonorrhoeae ATCC® 49226 | 0.06–0.25 | EUCAST/CLSI |
| Escherichia coli ATCC® 25922 | Not Applicable | Negative Control |
The QC data ensures consistent and reliable results for clinical and research use.
Regulatory and Validation Data
- CLSI Compliance: Meets the CLSI M07-A10 standards for MIC testing, ensuring compatibility with standardized susceptibility testing workflows.
- EUCAST Validation: Validated for use under EUCAST breakpoints and methodologies for Benzylpenicillin testing.
- ISO Compliance: Manufactured under ISO 20776-1 protocols for antimicrobial susceptibility testing systems.
- CE Marked: Certified for use in diagnostic applications across regions where CE compliance is required.
Comparison with Other Brands
| Feature | Liofilchem® ComASP™ Benzylpenicillin | Brand X | Brand Y |
|---|---|---|---|
| MIC Range | 0.002–32 µg/mL | 0.12–8 µg/mL | 0.06–16 µg/mL |
| Number of Tests | 12 per kit | 10 per kit | 8 per kit |
| Compliance | CLSI/EUCAST | EUCAST only | CLSI only |
| Cost-Effectiveness | High | Moderate | Moderate |
| Pathogen Coverage | Broad (Gram-positive) | Limited | Moderate |
Applications
- Clinical Diagnostics: Helps determine susceptibility of Streptococcus pneumoniae and Neisseria gonorrhoeae to Benzylpenicillin.
- Antimicrobial Resistance Research: Assists in tracking emerging resistance patterns.
- Pharmaceutical Development: Useful in evaluating efficacy during antibiotic development trials.
The Liofilchem® ComASP™ Benzylpenicillin MIC Test Panels offer a robust, validated solution for detecting bacterial susceptibility to Benzylpenicillin, ensuring high-quality results for clinical and research laboratories.




 0
0