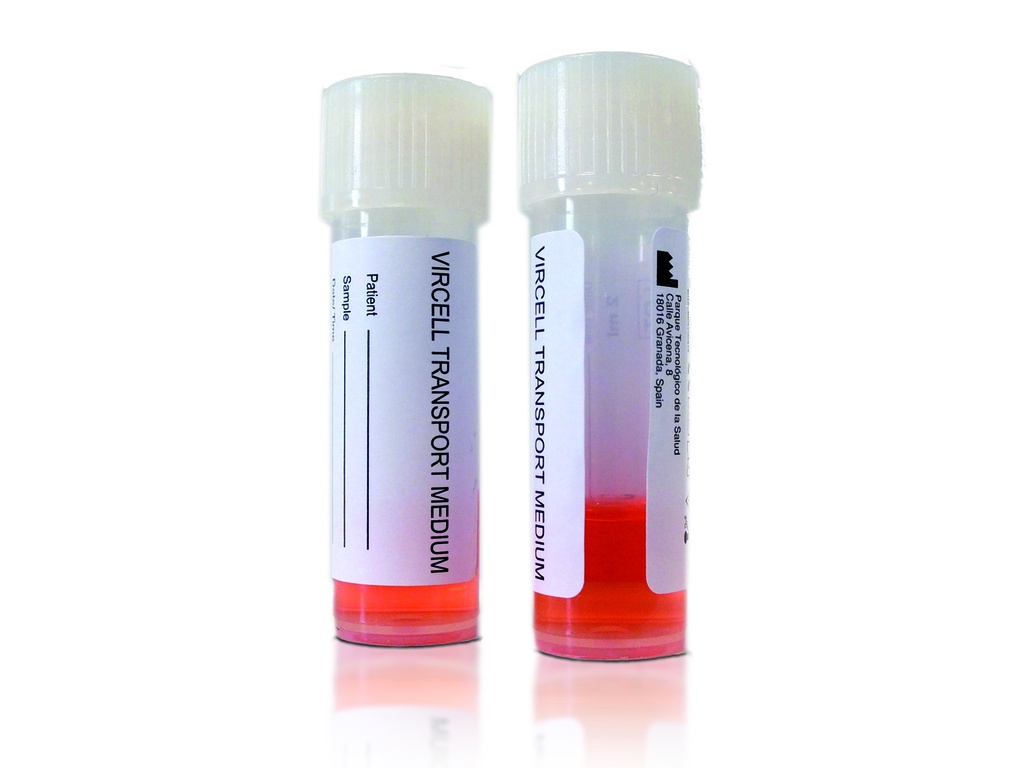
Specifications:
| Application | Sample Collection | ||
| Storage Temperature | Room Temperature | ||
| Product Type | sample collection, Culture Medium | Forms | Liquid |
| Product Brand | VIRCELL | ||
| Product Grade | Microbiology grade, Molecular Biology | ||
The Vircell Transport Medium (TM) is designed for the collection, transport, and preservation of Viruses, Chlamydia, and Mycoplasma from clinical specimens. Its optimized formulation prevents sample drying, maintains pathogen viability, and reduces bacterial overgrowth, ensuring high-quality specimens for molecular and culture-based diagnostics.
In addition to its general applications, the medium is also validated for RT-PCR detection of SARS-CoV-2 and Influenza A virus in saliva samples, providing a convenient and reliable solution for population screening programs. This unique format allows for self-sampling by patients, reducing the workload of healthcare professionals while maintaining diagnostic accuracy.
Key Features
- Versatile use – validated for viruses, Chlamydia, and Mycoplasma.
- Validated for SARS-CoV-2 & Influenza A (saliva samples) – comparable sensitivity to nasopharyngeal swabs in molecular assays.
- Self-sampling option – saliva collection device enables patient-driven collection, minimizing healthcare staff burden.
- Specimen preservation – prevents drying, maintains microbial viability, and reduces contamination.
- Molecular assay compatibility – proven performance with PCR/qPCR methods.
- Room temperature stability – simplifies transport and logistics.
- Unique barcode labeling – ensures accurate patient/sample traceability.
- Multiple swab and tube configurations – available with Dacron, Rayon, Nylon microfiber, or flocked swabs.
- CE-IVDR certified – compliance with international diagnostic standards.
Applications
- Collection and preservation of respiratory and urogenital specimens for viruses, Chlamydia, and Mycoplasma.
- Molecular diagnostics, including PCR/qPCR detection of SARS-CoV-2 and Influenza A virus from saliva samples.
- Large-scale population screening and epidemiological studies.
- Routine diagnostic use in hospitals, reference labs, and public health programs.
- Culture-based recovery of viable microorganisms.
- Long-distance transport of clinical specimens without cold chain.
Product Details
| Product Reference | Format | Volume | Swab/Collection Device | Pack Size | Class |
|---|---|---|---|---|---|
| TM004 | Bottle | 100 mL, 53×108 mm | No swab | – | CE-IVDR |
| TM003 | Tube | 2 mL, 16.5×57 mm | 1 sterile Dacron swab | 50 | CE-IVDR |
| TM011 | Tube | 2 mL, 16.5×57 mm | 1 sterile Rayon swab | 50 | CE-IVDR |
| TM012 | Tube | 2 mL, 16.5×57 mm | 1 sterile Nylon microfiber swab | 50 | CE-IVDR |
| TM006 | Tube | 2 mL, 16.5×57 mm | 2 sterile Dacron swabs | 50 | CE-IVDR |
| TM005 | Tube | 2 mL, 16.5×57 mm | No swab | 50 | CE-IVDR |
| TM018 | Tube | 2 mL, 16×100 mm | 1 sterile mini-tip flocked swab | 50 | CE-IVDR |
| TM014 | Tube | 2 mL, 16×100 mm | 1 sterile Nylon microfiber swab | 50 | CE-IVDR |
| TM001 | Tube | 2 mL, 16.5×57 mm | 2 sterile Rayon swabs | 50 | CE-IVDR |
| TM020 | Tube | 2 mL, 16×100 mm | 2 swabs (Nylon microfiber, mini-tip flocked) | 50 | CE-IVDR |
| TM010 | Tube | 2 mL, 16×100 mm | 2 swabs (Nylon microfiber, Rayon) | 50 | CE-IVDR |
| TM022 | Tube | 3 mL, 16×100 mm | 2 swabs (Nylon microfiber, Rayon) | 50 | CE-IVDR |
| TM008 | Tube | 3 mL, 16.5×57 mm | 1 sterile Nylon microfiber swab | 50 | CE-IVDR |
| TM016 | Tube | 3 mL, 16×100 mm | 1 sterile Nylon microfiber swab | 50 | CE-IVDR |
| TM021 | Tube | 3 mL, 16×100 mm | No swab | 50 | CE-IVDR |
| TM002 | Tube | 3 mL, 16.5×57 mm | No swab | 50 | CE-IVDR |
| TM015 | Tube | 1 mL, 16×100 mm | Saliva collection device | 50 | CE-IVDR |
Kit Contents
- Vircell Transport Medium in screw-cap bottles or tubes.
- Configurations with 0–2 sterile swabs (Dacron, Rayon, Nylon microfiber, flocked, or mixed).
- Special saliva collection device (TM015) for SARS-CoV-2 and Influenza A testing.
- Formulation: Hank’s BSS with HEPES, gelatin, BSA, sucrose, and antibiotics.
The Vircell Transport Medium provides laboratories with a flexible, CE-IVDR certified solution for specimen collection, preservation, and transport. With options for standard swab-based sampling or saliva-based collection for SARS-CoV-2 and Influenza A, it ensures accurate diagnostics, streamlined logistics, and compliance with international standards.
- Description: Bottle, no swab, 100 mL x 1 Tube, 2 mL with 1 sterile Dacron swab, 50/pk Tube, 2 mL with 1 sterile Rayon swab, 50/pk Tube, 2 mL with 1 sterile Nylon microfiber swab, 50/pk Tube, 2 mL with 2 sterile Dacron swabs, 50/pk Tube, 16.5×57 mm, 2 mL without swab, 50/pk Tube, 16×100 mm, 2 mL with 1 sterile mini-tip flocked swab, 50/pk Tube, 16×100 mm, 2 mL with 1 sterile Nylon microfiber swab, 50/pk Tube, 16.5×57 mm, 2 mL with 2 sterile Rayon swabs, 50/pk Tube, 16×100 mm, 2 mL with 2 sterile swabs (Nylon microfiber, mini-tip flocked), 50/pk Tube, 16×100 mm, 2 mL VTM with 2 sterile swabs (Nylon microfiber, Rayon), 50/pk Tube, 16×100 mm, 3 mL VTM with 2 swabs (Nylon microfiber, Rayon), 50/pk Tube, 16.5×57 mm, 3 mL VTM with 1 sterile Nylon microfiber swab, 50/pk Tube, 16×100 mm, 3 mL VTM with 1 sterile Nylon microfiber swab, 50/pk Tube, 16×100 mm, 3 mL VTM, No swab included, 50/pk Tube, 16.5×57 mm, 3 mL VTM, No swab included, 50/pk Tube, 16×100 mm, 1 mL VTM with Saliva collection device, 50/pk




 0
0